Meaningful Use Stage 2 | A View on Penalties and Repercussions | Part Two
The second part in our two-part series: Meaningful Use 2 - A View on Penalties and Repercussions. The series takes a in-depth and comprehensive look at the background of Meaningful Use, as well as the measures and objectives of MU Stage 1 and Stage 2. Click here to read part one
At this point, many providers have either started or attested for Stage 1 of the Meaningful Use EHR Incentive Program. Stage 2 is scheduled to begin in 2014. In Stage 1, eligible providers (EPs) had to meet 15 core objectives and five of 10 menu objectives. EPs must meet 17 core objectives and three of six menu objectives for Stage 2.
The final number of measures EPs must satisfy remains the same in Stage 2; however, the requirements become more detailed and coordinated. Nearly all of the Stage 1 core and menu objectives are retained.
Most of the change is in the increase in the percentages for measures that providers must comply with and many of the Stage 1 measures combine to make up Stage 2 measures.
- Stage 2 of Meaningful Use includes more patient engagement and information exchange requirements.
Stage 2 Measures as described by the Centers for Medicare and Medicaid Services (CMS) as follows:
Stage 2 Measures
Stage 2 MU Core Objectives: EPs must report on all 17 core objectives
- Use computerized provider order entry (CPOE) for medication, laboratory and radiology orders
- Generate and transmit permissible prescriptions electronically (eRx)
- Record demographic information
- Record and chart changes in vital signs
- Record smoking status for patients 13 years old or older
- Use clinical decision support to improve performance on high-priority health conditions
- Provide patients the ability to view online, download and transmit their health information
- Provide clinical summaries for patients for each office visit
- Protect electronic health information created or maintained by the Certified EHR Technology (CEHRT)
- Incorporate clinical lab-test results into Certified EHR Technology
- Generate lists of patients by specific conditions to use for quality improvement, reduction of disparities, research, or outreach
- Use clinically relevant information to identify patients who should receive reminders for preventive/follow-up care
- Use certified EHR technology to identify patient-specific education resources
- Perform medication reconciliation
- Provide summary of care record for each transition of care or referral
- Submit electronic data to immunization registries
- Use secure electronic messaging to communicate with patients on relevant health information
Stage 2 MU Menu Objectives: EPs must also report on 3 of these 6 menu objectives
- Submit electronic syndromic surveillance data to public health agencies
- Record electronic notes in patient records
- Imaging results accessible through CEHRT
- Record patient family health history
- Identify and report cancer cases to a State cancer registry
- Identify and report specific cases to a specialized registry (other than a cancer registry).
Stage 1 versus Stage 2
Clinical Quality Measures (CQM)
EPs will submit 9 CQMs from at least 3 of the National Quality Strategy domains out of a potential list of 64 CQMs across 6 domains under the Stage 2 rules.
In Stage 1, EPs must report three core CQMs (or if the denominator of one or more of those core measures is zero, then eligible professionals may report up to three alternate core measures). In the first stage, EPs must also report an additional three of 38 measures.
Stage 2 CQMs somewhat parallel with pre-existing national quality programs, such as measures used for Physician Quality Reporting System (PQRS), Accountable Care Organizations (ACOs), and Patient-Centered Medical Homes (PCMHs).
Core Measures
1. Computerized provider order entry (CPOE) for medication orders
Stage 2: Record using CPOE more than 60 percent of medication, 30 percent of laboratory, and 30 percent radiology orders created by the EP.
Stage 1: Record using CPOE more than 30 percent of unique patients with at least one medication in their medication list (also only applied to medication orders).
2. Generate and transmit permissible prescriptions electronically (e-Rx)
Stage 2: More than 50 percent of all permissible prescriptions, or all prescriptions written by the EP, are queried for a drug formulary and transmitted electronically using CEHRT.
Stage 1: More than 40 percent of all permissible prescriptions written by the EP are transmitted electronically using certified EHR technology (comparison to drug formulary is not included)
3. Record demographics
Stage 2: More than 80 percent of all unique patients seen by the EP during the EHR reporting period have demographics recorded as structured data.
Stage 1: More than 50 percent of all unique patients seen by the EP during the EHR reporting period have demographics recorded as structured data.
4. Record vital signs
Stage 2: More than 80 percent of all unique patients seen by the EP during the EHR reporting period have blood pressure (for patients age 3 and over only) and height/length and weight (for all ages) recorded as structured data.
Stage 1: More than 50 percent of all unique patients age 2 and over seen by the EP have height, weight, and blood pressure recorded as structured data.
5. Record smoking status
Stage 2: The EP records, for more than 80 percent of all unique patients age 13 or older, smoking status recorded as structured data.
Stage 1: The EP records, for more than 50 percent of all unique patients age 13 or older, smoking status recorded as structured data.
6. Report ambulatory clinical quality measures
Stage 2: The EP implements five clinical decision support interventions related to five or more clinical quality measures; and the EP enables and implements the functionality for drug-drug and drug-allergy interaction checks for the entire EHR reporting period.
Stage 1: The EP successfully reports ambulatory clinical quality measures selected by CMS in the manner specified (or in the case of Medicaid, the States) and implements one clinical decision support rule; and the EP implements the functionality for drug-drug and drug-allergy interaction checks for the entire EHR reporting period.
7. Incorporate clinical lab results
Stage 2: More than 55 percent of all clinical lab tests results ordered by the EP are incorporated in Certified EHR Technology as structured data.
Stage 1: More than 40 percent of all clinical lab tests results ordered by the EP are incorporated in certified EHR technology as structured data. This is optional and appears in the menu set for Stage 1.
8. Detail specific patient conditions
Stage 2: Generate at least one report listing patients of the EP with a specific condition.
Stage 1: This is optional and appears in the menu set.
9. Patient reminders
Stage 2: More than 10 percent of all unique patients who have had an office visit with the EP within 24 months prior to the beginning of the EHR reporting period are sent a reminder, per patient preference.
Stage 1: More than 20 percent of patients older than 75 or younger than 5 are sent reminders for preventive follow-up care. This is optional and appears in the menu set.
10. Patient access to health information
Stage 2: More than 50 percent of all unique patients seen by the EP during the EHR reporting period are provided online access to their health information within four business days after the information is available to the EP, subject to the EP’s discretion to withhold certain information. And, more than 5 percent of all unique patients seen by the EP (or their authorized representatives) view, download, or transmit to a third party their health information within four business days.
Stage 1: This objective replaces the Stage 1 core objective for EPs of “Provide patients with an electronic copy of their health information upon request” and the Stage 1 menu objective of “Provide patients with timely electronic access to their health information (including lab results, problem list, medication lists, and allergies) within four business days of the information being available to the EP.”
11. Provide clinical summaries to patients
Stage 2: The EP provides clinical summaries to patients within 1 business day for more than 50 percent of office visits.
Stage 1: The EP provides, for more than 50 percent of all patients who request it, an electronic copy of their clinical summaries within three business days.
12. Provide patient-specific educational resources
Stage 2: The EP provides, for more than 10 percent of all office visits, patient- specific education resources identified by EHR.
Stage 1: More than 10 percent of all unique patients seen by the EP receive patient-specific resources. This is optional and appears in the menu set.
13. Transitions of care
Stage 2: The EP performs medication reconciliation for more than 50 percent of transitions of care in which the patient is transitioned into the care of the EP.
Stage 1: The EP performs medication reconciliation for more than 50 percent of all transitions of care in which the patient is transitioned into the care of the EP. This is optional and appears in the menu set.
14. Provide summary of care records
Stage 2: The EP provides a summary of care record for more than 50 percent of transitions of care and referrals. And the EP provides a summary of care record for more than 10 percent transitions and referrals either electronically transmitted using CEHRT to a recipient or where the recipient receives the summary of care record via exchange facilitated by an organization that is a NHIN/NwHIN Exchange participant or in a manner that is consistent with the governance mechanism ONC establishes for the nationwide health information network. And, an EP, must satisfy one of the two following criteria: 1. Conduct one or more successful electronic exchanges of a summary of care document with a recipient who has EHR technology that was developed designed by a different EHR technology developer than the sender's EHR; 2. Conduct one or more successful tests with the CMS designated test EHR during the EHR reporting period.
Stage 1: The EP provides a summary of care record for more than 50 percent of transitions of care and referrals. This is optional and not required. The second aspect of the Stage 2 requirement, transmit a summary of care record electronically to a recipient using a different EHR, is new.
15. Provide immunization data
Stage 2: The EP successfully submits electronic immunization data from the EHR to an immunization registry or immunization information system for the entire EHR reporting period.
Stage 1: The EP submits electronic immunization data from the EHR to an immunization registry or immunization information system; does not require successful submission. This is optional and appears in the menu set.
16. Protect electronic health information
Stage 2: The EP conducts or reviews a security risk analysis in accordance with the requirements under 45 CFR 164.308(a)(1), including addressing the encryption/security of data in accordance with requirements under 45 CFR
Stage 1: The above Stage 2 measure is almost the same as in Stage 1.
17. Secure messaging to patients
Stage 2: More than 5 percent of unique patients seen during the reporting period send a secure message using the electronic messaging function of the EHR.
Stage 1: Does not appear.
Menu Set Measures in Stage 2
The following are Menu Set measures that that do not appear in Stage 1 but now appear in Stage 2.
1. Scans and tests
Stage 2: More than 10 percent of all scans and tests ordered by the EP that result in an image are accessible through the EHR.
2. Family history
Stage 2: More than 20 percent of all unique patients seen by the EP have a structured data entry for one or more first-degree relatives (a family member who shares about 50 percent of their genes with a particular patient including the patient’s parents, siblings, and children).
3. Electronic syndromic surveillance data
Stage 2: Successful ongoing submission of electronic syndromic surveillance data from the EHR to a public health agency for the entire EHR reporting period. (In Stage 1, this is a single test and does not require successful ongoing submission.)
4. Cancer case information
Stage 2: Successful ongoing submission of cancer case information from a certified EHR to a cancer registry for the entire EHR reporting period.
5. Specific case information
Stage 2: Successful ongoing submission of specific case information from a certified EHR to a specialized registry for the entire EHR reporting period.
6. Electronic notes
Stage 2: Enter at least one electronic progress note created, edited, and signed by an EP for more than 30 percent of unique patients with at least one office visit during the EHR reporting period.
Stage 1 Objectives Eliminated from Stage 2
1. Exchange of clinical information
The “exchange of key clinical information” core objective from Stage 1 is replaced by a ‘‘transitions of care’’ core objective in Stage 2.
2. Electronic copies
The “provide patients with an electronic copy of their health information” objective is replaced by an ‘‘electronic/online access’’ core objective in Stage 2.
Repercussions and Penalties
Timing of Stage 2
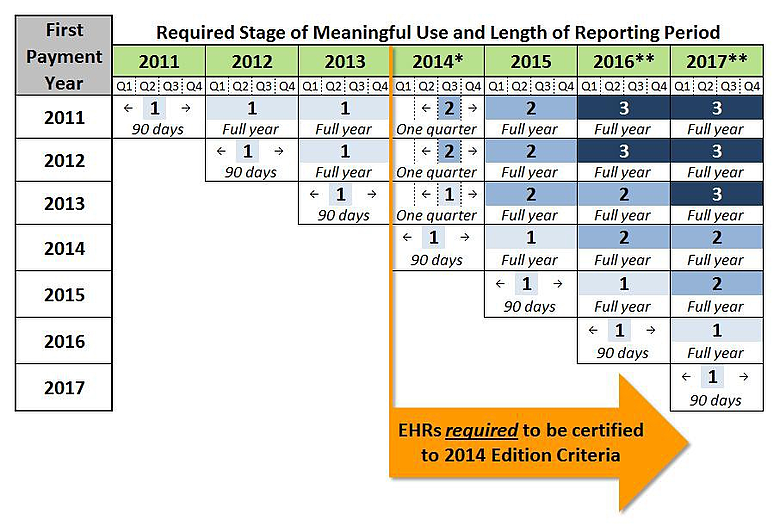
Eligible hospitals (EHs) and eligible professionals (EPs) who attested in 2011 will get three years on Stage 1, while all other EHs and EPs (regardless of when their first payment year is) will have two years on Stage 1 before moving to Stage 2. CMS reiterated in the Stage 2 Rule that Stage 3 remains on track for 2016, and additional stages are not out of the question in future years.
Perhaps the most unexpected change in the Stage 2 Rule relates to more flexibility in reporting periods for the 2014 payment year. Since all EHs and EPs (regardless of whether they are on Stage 1 or Stage 2) will be required to have EHRs tested against 2014 Edition certification criteria by the start of the 2014 reporting period, CMS is essentially giving providers that have previously attested up to nine additional months to test and implement newly-certified EHR capabilities.
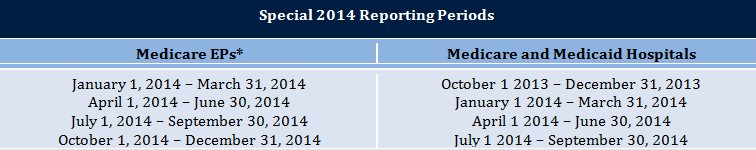
*States will decide if the reporting period for Medicaid EPs is one 3 month quarter or any consecutive 90-day period
The 2014 reporting period for EHs and Medicare EPs on their second year of Stage 1 or their first year of Stage 2 will now be a three-month quarter rather than a full year. (See the table above for allowable reporting periods.) This quarterly approach was adopted to balance the demand of meeting new EHR certification requirements with the need to preserve some alignment with other federal quality reporting programs, such as PQRS and the Medicare Shared Savings Program. To avoid undue complexity for state Medicaid agencies, each state will be able to decide whether Medicaid EPs who have previously attested will also have a quarter-long reporting period for 2014, or if EPs can select any 90-consecutive-day reporting period during the payment year. Note the changes to the 2014 reporting period do not apply to EHs and EPs attesting to meaningful use for the first time in 2014; those providers will continue to have a 90-consecutive-day reporting period (anytime in the payment year).
The following chart outlines required Stages of meaningful use and the length of each associated reporting period. (Note the EH program is based on Federal Fiscal Years; the EP program is based on calendar years.)
*States will decide whether the reporting period for Medicaid EPs attesting to meaningful use in 2014 will be one quarter or any 90-consecutive-day period.
**Details of Stage 3 in 2016 and beyond are subject to future rulemaking
Penalties and Exemptions
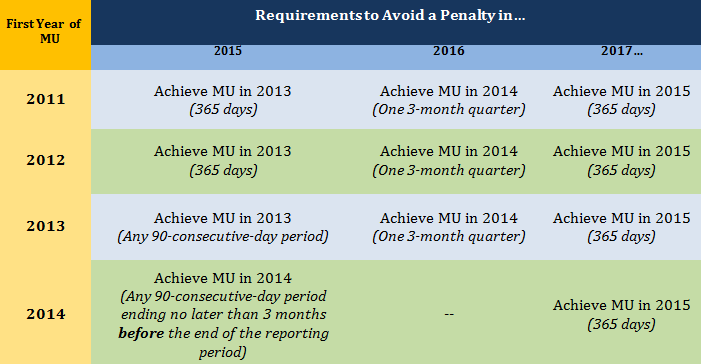
The Stage 2 Rule also establishes the timeline for how reductions in Medicare reimbursement will be calculated for EHs and EPs who fail to meet meaningful use requirements for a given year. Although the annual penalties will not begin until 2015, they will actually be based on performance two years earlier. In other words, to avoid the penalty in 2015, an EH or EP that successfully attests in 2012 would need to meet meaningful use for the 2013 payment year. To avoid the penalty in 2016, the EH or EP would need to achieve meaningful use in 2014.
CMS makes an exception for EHs and EPs whose first year of meaningful use is 2014. To avoid the penalty in 2015, these providers will need to successfully attest at least three months before the end of the 2014 payment year. For EPs whose first payment year is 2014, this means the 90-consecutive-day reporting period needs to begin no later than July 3, 2014 to avoid the penalty in 2015; for EHs attesting for the first time in 2014, the reporting period will need to begin no later than April 2, 2014.
The following chart outlines the requirements needed to avoid a penalty in 2015, 2016 and 2017. (Note that penalties continue in 2018 and all subsequent years.)
Certain EHs and EPs may qualify for a temporary reprieve from penalties in a given year if CMS determines meeting meaningful use requirements would result in “significant hardship.” Although the Stage 2 Rule outlines a number of possible types of exemptions (insufficient Internet access, recently opened hospitals, practices affected by a natural disaster, etc.), there are no guarantees; all exemptions will be reviewed annually on a case-by-case basis, and in no instance may an exemption be granted for more than five years. Also, any exemption applies to events and circumstances that occur in the performance year, not the penalty year.
- Meeting meaningful use is an annual and ongoing event. The timing of penalties significantly raises the consequences of missing a payment year. Medicare EPs and EHs that successfully attest in 2012 but fail to meet requirements for 2013 will not only lose their 2013 incentive payment; they will also be subject to the penalty in 2015. While Medicaid EPs and EHs can receive an initial incentive payment for “adopting, implementing, or upgrading” Certified EHR Technology, if they fail to actually demonstrate meaningful use at least three months before the end of the 2014 payment year, the penalty in 2015 will apply.
- 2014 Readiness. Although CMS has given providers that previously attested in 2011, 2012 or 2013 as much as nine additional months of breathing room to meet 2014 requirements, organizations, EHs and EPs should be proactively working with their vendors to ensure a plan is in place to implement and test new EHR capabilities and make any necessary adjustments to existing workflow and reporting.
Conclusion
Since its launch in January 2011 through July 2012, approximately 117,000 eligible professionals and 3600 hospitals have received incentive funds (CMS, 2012). Essentially Stage 1 triggered the simple adoption, use and data capture through CEHRT.
Stage 2 now begins to leverage the data contained therein to engage the patient, an essential shift in the paradigm that is vital to the mission of improving quality and healthcare outcomes and potentially lower healthcare costs. Stage 2 also begins to break down the walls between providers through data sharing to improve collaboration with the undoubted returns in quality and costs. Finally, Stage 2 also automates the process of clinical quality reporting. This reporting of healthcare data is foundational to efforts underway in payment reform.
Meaningful Use 2 | A View on Penalties and Repercussions | Part One